The Canadian National Crystallographic Committee will be hosting the fourth Dr. Penelope W. Codding Lecture in support of early career crystallographers on Wednesday, the 20th of December, at 9:30 a.m. Pacific time/12:30 p.m. Eastern time.
Title: Unlocking ATP Synthase’s New Horizons through Theoretical & Computational Analysis
Abstract:
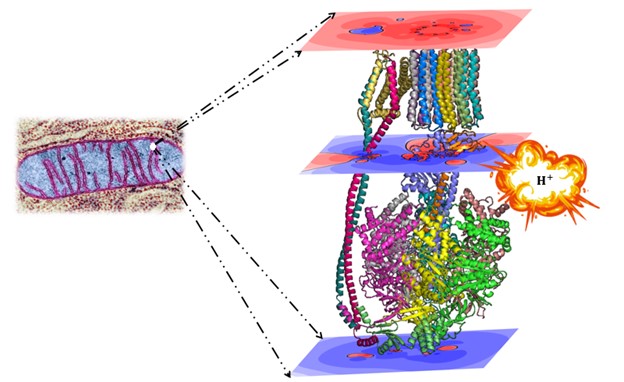
ATP synthase serves as the pivotal nexus for ATP production, the essential energy currency in living systems. The structure of this crucial enzyme has been recently determined by X-ray crystallography and electron microscopy. Using these structures as the basis to study the function of this enzyme, the enzyme appears to have novel roles, never previously reported, in bioenergetics and thermodynamics. Our findings include:
1) A non-negligible contribution to chemiosmotic energy due to the intrinsic electrostatic potential of ATP synthase itself (nothing to do with the textbook well-known chemiosmotic energy stored in the proton imbalance across the inner mitochondrial (or bacterial) membrane). At least in the 5 studied structures, this may introduce a new term in the equation central to Mitchell’s chemiosmotic theory [1].
2) Identifying a second new role for ATP synthase as a regulator of the rate (kinetic barrier) for the flow of protons through the enzyme [1] and hence of the power (rate for energy flow) of ATP synthase [2,3].
3) Formulating a theory for the enzyme’s remarkably low thermal conductivity from an analogy to a ratchet engine [4].
4) Proposing a possible resolution to a million-fold disagreement between experiment and theory concerning the temperature of mitochondrial operation. This proposal demonstrates that each proton translocated through the enzyme generates a substantial but short-lived temperature gradient, lasting on the order of picoseconds. This gradient aligns with the experimental reports, and when averaged over time, it recovers the theoretical predictions, resolving this apparent paradox [4,5].
References:
[1] J.-N. Vigneau, P. Fahimi, M. Ebert, Y. Cheng, C. Tannahill, P. Muir, T.T. Nguyen-Dang, C.F. Matta, ATP synthase: A moonlighting enzyme with unprecedented functions, Chem. Commun. 58 (2022) 2650–2653.
[2] P. Fahimi, C.F. Matta, J.G. Okie, Are size and mitochondrial power of cells inter-determined?, J. Theor. Biol. 572 (2023) Article # 111565.
[3] P. Fahimi, C.F. Matta, On the power per mitochondrion and the number of associated active ATP synthases, Phys. Biol. 18 (2021) Article # 04LT01.
[4] P. Fahimi, C.F. Matta, The hot mitochondrion paradox: Reconciling theory and experiment, Trends Chem. 4 (2022) 96–110.
[5] P. Fahimi, M.A. Nasr, L.M. Castanedo, Y. Cheng, C.A. Toussi, C.F. Matta, A note on the consequences of a hot mitochondrion: Some recent developments and open questions, Biophys. Bull. 43 (2020) 14–21.
Biography: Peyman Fahimi earned his B.Sc. in Physics from Bu-Ali Sina University in 2012 and subsequently completed his M.Sc. in Physics at Khajeh Nasir Toosi University of Technology in 2017, both in Iran. He later joined the Department of Chemistry at Université Laval, where he just got his PhD in 2023 under the supervision of Professor Chérif F. Matta and Professor Thanh-Tung Nguyen-Dang. His doctoral thesis is titled “Theoretical Investigations in Mitochondrial Biophysics”, which contains among its main topics the above-mentioned resolution of the controversy concerning the temperature of operation of the mitochondria. Recently, Peyman accepted a postdoctoral fellowship in the Department of Mathematics and Statistics at Dalhousie University. In collaboration with Professor Andrew J. Irwin and Professor Zoe Finkel, he will be working on developing models for phytoplankton growth and elemental composition. Peyman’s overarching research objective is to contribute to an integrative understanding of living systems across scale from biomolecules to organelles, cells, and microorganisms.
